Types of Distillation – Distillation is a unit process widely used operation in chemical industries all over the world to separate components of a mixture. Distillation is a process of liquid evaporation converted into vapor and recondensed and collected in a vessel.
In the manufacturing process, distillation is a commonly used unit operation as a part of the solvent recovery process (SR Plant), impurity removal process or purification process, and many more. Today, we are going to learn Types of Distillation and the definition of the distillation process.
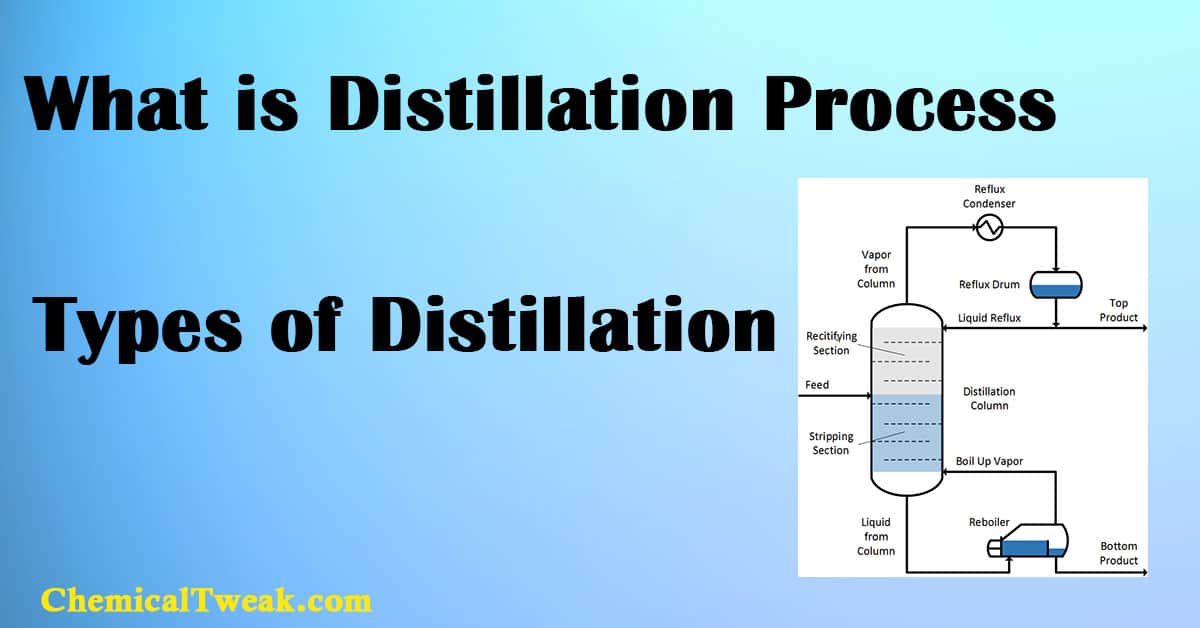
Table of Contents
What is Distillation mean?
Distillation can be defined as the process of separating components or substances from a mixture using different boiling points or relative volatility. In other words, the distillation Process involves the conversion of a liquid into a vapor that is subsequently condensed back to liquid form and thereby separates one liquid from another. Learn what are unit operation and unit process.
Different types of Distillation
- Simple distillation
- Steam Distillation
- Vacuum Distillation
- Fractional Distillation
- Azeotropic Distillation
- Extractive Distillation
Let us have a simple introduction to all the different types and the working principle.
1. Simple Distillation
Simple distillation is the basic type of distillation process in which a liquid mixture of two components is heated until the substance with a lower boiling point starts vaporizing. The vapor is then condensed with the help of a condenser with suitable utility, and then collected in another vessel. This process is used to separate the mixture to is a wide boiling point difference of more than 25 degrees or more.
2. Steam Distillation

Simple distillation is the basic type of process in which steam is introduced to the mixture which heats the liquid mixture, which will increase the vapor pressure of the components. When the vapor pressure of the immiscible components exceeds the atmospheric pressure, the high-boiling component will start evaporating at low temperatures and form a mixture with water.
Steam distillation is used to separate out the heat-sensitive components which can decompose at high temperatures. A common example of Steam distillation is it is used to extract oil from plant matter.
3. Vacuum Distillation
The distillation process can be classified as atmospheric and vacuum distillation. The process, which is conducted under vacuum or negative pressure, is known as vacuum distillation. It is similar to atmospheric, but the only difference is that vacuum distillation is conducted under vacuum. The boiling point is directly proportional to pressure.
If the boiling point of any substance present in the mixture is high, in that case, we need to use a utility that can help to achieve the boiling point of that high-boiling component which will be costly. So to avoid using that utility, vacuum distillation is used in which a vacuum is created in the column which will decrease the boiling point of the component, and hence separation can be achieved at a low temperature.
4. Fractional Distillation
The principle behind fractional distillation is that different components in the mixtures boil at different temperatures. In fractional distillation, so the mixture is heated and the low-boiling substance starts evaporating first, condenses the liquid first, and separates out. Now, increase the temperature and similarly separate out the components from the lower boiling point to the higher boiling point.
This is commonly carried out in refineries where the distillation of crude oil is carried out. Different components like petrol, diesel, kerosene, naptha are separated from the crude oil using fractional distillation as the boiling points of these substances present in the crude oil are different.
5. Azeotropic distillation
Azeotropic distillation is carried out when we have a mixture of immiscible liquid which cannot be separated using simple distillation as the difference in boiling point is very low and such mixtures are called an azeotropic mixture. If we carried out the simple distillation of the azeotropic mixture, vapor generated by heating the mixture will contain both the component, hence separation though the simple distillation method can’t be done. In this case, the Azeotropic distillation process is carried out.
In the Azeotropic distillation method, in the mixture of supposing A and B, an additional substance i.e., C is added which has the ability to alter the activity coefficient of the components and thereby change the relative volatility of the mixture. The third component added to the mixture is known as an entertainer, which will create either minimum-boiling point with one of the components.
This was the introduction, We covered this topic individually and in detail. If you want to learn in detail, along with example,s then you can read the Azeotropic Distillation process in detail along with examples.
6. Extractive Distillation
Extractive Distillation can be defined as process performed in the presence of a miscible, high-boiling, relatively non-volatile component, the solvent, that forms no azeotrope with the other components in the mixture. This method is used for mixtures having a low value of relative volatility, nearing unity. In this method, an additional component called entrainer is added to the mixture but it will not form any azeotrope but act differently with the component of the mixture and cause a change in relative volatility to allow the new three-part mixture to be separated by normal distillation.
💡 Want to visualize distillation stages? Try our free McCabe-Thiele Calculator to understand the number of theoretical plates needed for separation.
FAQ (Different types of distillation process)
1. How many types of distillation are there?
Ans – There are a total of 6 types, and they are Simple, Steam , Vacuum , Fractional, Azeotropic, and Extractive Distillation
2. What are the three steps of distillation?
Distillation is a three-step process: heating the liquid to change the phase to vapor form, condensing the liquid, and collecting the condensed liquid.
3. How many types of distillation are there in the pharmaceutical industry?
In the pharmaceutical industry, types of the distillation process is used are simple, extractive , azeotropic, and vacuum distillation. Fractional distillation is not used in the pharmaceutical industry as it is only used in petroleum or refinery.
2. What is the use of distillation?
The process of distillation can be used to purify liquids or to separate them into their individual components.
This is used in a variety of industries, including refineries and in the production of alcohol, essential oils, and purified water.
Wrapping Up
This was a simple introduction article on what is Distillation and the different types. We will be covering different types in detail so stay tuned with us. If you are a chemical engineering student or working professional, or in case you want to have chemical engineering knowledge, check out our blog, we had covered various articles related to chemical engineering and Industrial safety.

Distillation process is easily understand
Thanks
I no I did not do well but I will keep on learning
Thanks
Your welcome..!!